You are here
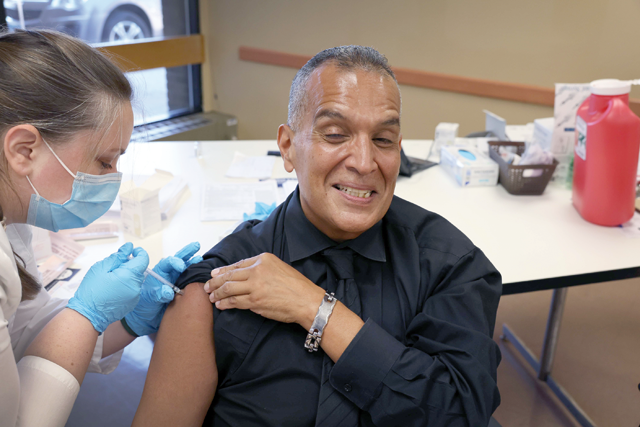
Sweden halts use of Moderna vaccine for young adults
By AFP - Oct 06,2021 - Last updated at Oct 06,2021
STOCKHOLM — Sweden’s Public Health Agency on Wednesday recommended a temporary halt to the use of the Moderna COVID-19 vaccine among young adults, citing concerns over rare side effects to the heart.
Neighbouring Norway and Denmark also on Wednesday reiterated that Moderna’s vaccine was not recommended for under-18s.
Sweden’s health agency said the suspension concerned anyone born after 1991 and should initially be in force until December 1, explaining that it had received evidence of an increased risk of side effects such as inflammation of the heart muscle (myocarditis) and inflammation of the pericardium (pericarditis).
“The Public Health Agency has decided to pause the use of Moderna’s vaccine Spikevax, for everyone born 1991 and after, for cautionary reasons,” the agency said in a statement, adding that those groups should instead receive the Pfizer/BioNTech vaccine.
According to the agency, the risk seems especially tied to the second dose of the Moderna vaccine and is more prevalent among young men and boys, and in the weeks just following the second jab.
The symptoms usually pass by themselves, but should be evaluated by a doctor, it added.
“Those who were vaccinated recently, with their first or second dose of Moderna’s vaccine, don’t need to feel worried because the risk is very minor, but it is good to know which symptoms you should be on the lookout for,” state epidemiologist Anders Tegnell said in a statement.
The health agency’s recommendations provide guidance for Sweden’s regional healthcare districts and are generally followed.
Some 81,000 people in the age group have already received a first jab in Sweden, but they will not be offered a second, the agency said, adding that “discussions were ongoing about the best solution for this group”.
In Denmark, the vaccination programme for those aged 12 to 17 currently only offers Pfizer/BioNTech’s Comirnaty vaccine but the country’s health authority said on Wednesday this practice would be formalised.
“On the basis of the precautionary principle, we will in future invite children and adolescents to receive this vaccine exclusively,” Bolette Soborg of the healthy said in a statement, noting the availability of more data on the use among younger people for the Pfizer/BioNTech vaccine.
The Norwegian Institute of Public Health (NIPH) reiterated its recommendation to vaccinate under-18s only with Comirnaty, but added on Wednesday that men under 30 should also consider choosing the Pfizer/BioNTech jab when getting vaccinated.
“It is important to emphasise that Spikevax from Moderna is as effective a vaccine against COVID-19 as Comirnaty, and both types of vaccine are still recommended for those over 30 years of age,” Geir Bukholm, deputy director general at NIPH, said in a statement.
The European Medicines Agency authorised the emergency use of Moderna’s COVID-19 vaccine for teens in July.
Related Articles
FRANKFURT AM MAIN — Europe's medicines regulator said Tuesday it would decide by December 29 whether to grant emergency approval to a COVID-
THE HAGUE — The EU’s medicines watchdog on Monday approved a vaccine specifically targeting the new and contagious types of the Omicron vari
PARIS — France became the latest country on Tuesday to authorise new COVID-19 vaccines that have been updated to target Omicron subvariants