You are here
Heart attack treatment gel safe for humans
By The San Diego Union-Tribune (TNS) - Sep 19,2019 - Last updated at Sep 19,2019

AFP photo
By Bradley J. Fikes
SAN DIEGO — A heart attack treatment from San Diego researchers has shown evidence of safety in a human study, along with early signs that it might be effective.
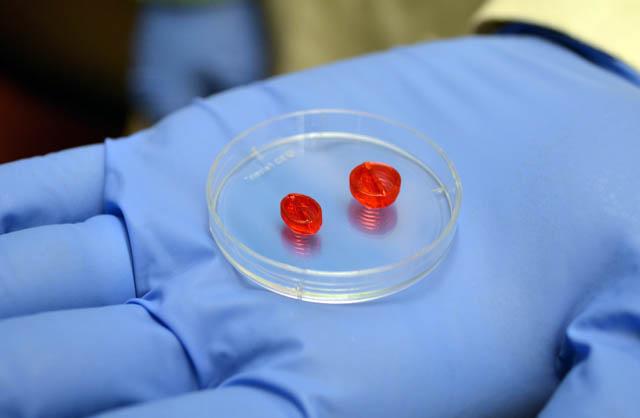
The treatment from the company Ventrix is a liquid that turns into a gel when injected into the heart. It provides a scaffold for new cells to take hold and repair the heart.
By encouraging growth of new muscle, the treatment, called VentriGel, is intended to reduce scar formation and increase cardiac muscle. Since scar tissue doesn’t contract, the burden of pumping is increased for the rest of the heart. Over time, the heart enlarges and begins to pump less efficiently, causing heart failure.
The University of California, San Diego spinoff reported the results last week in Journal of the American College of Cardiology: Basic to Translational Science. A 2013 study in pigs showed that VentriGel can improve performance of damaged hearts.
VentriGel contains a variety of large molecules, collectively known as the extracellular matrix, that support cells. It’s made from connective tissue take from pig hearts, which are treated to remove all cells. What’s left is the extracellular matrix, said study author Karen Christman, a UC San Diego professor of bioengineering at the Jacobs School of Engineering. She is also a co-founder of Ventrix.
VentriGel provides a porous and fibrous structure for new cells to latch onto, Christman said. The human immune system accepts VentriGel as is, meaning that immune-suppressing drugs aren’t needed.
The Phase 1 or early-stage trial treated 15 patients, 12 of them men, who had mild to moderate heart failure following a heart attack. Patients received the gel via injections with a cardiac catheter in a minimally invasive procedure similar to how a stent would be inserted.
“Patients in general were feeling better, they could walk further,” Christman said. And heart size decreased for those who had had a heart attack more than one year before treatment.
However, patients knew they were getting VentriGel, so the placebo effect can’t be ruled out. A larger controlled study will be needed to provide more reliable evidence of efficacy.
Ventrix is seeking about $20 million to do that midstage trial, Christman said.
“Ventrix has designed a Phase 2 clinical study and has completed preparations for starting the next phase in the beginning of next year,” she said.
Related Articles
WASHINGTON — US scientists have developed a new form of drug that promotes the regeneration of cells and reversed paralysis in mice with spi
It may sound far-fetched, but scientists are attempting to build a human heart with a 3-D printer.
The Vesugen peptide, derived from vascular endothelial tissues, has emerged as a promising subject of research due to its unique structural