You are here
Results show COVID vaccine 95% effective
By AFP - Nov 18,2020 - Last updated at Nov 18,2020
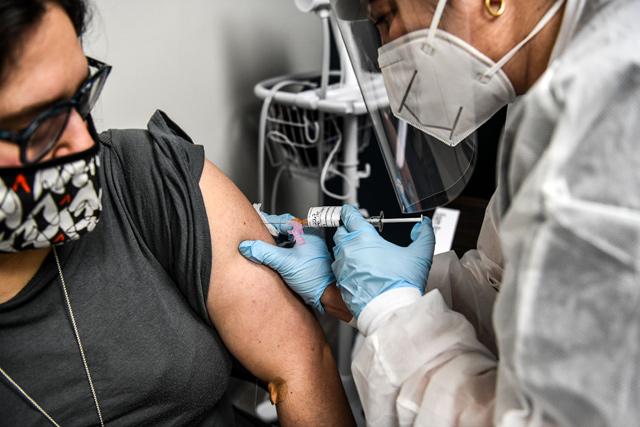
In this file photo Heather Lieberman (left), 28, receives a COVID-19 vaccination from Yaquelin De La Cruz at the Research Centres of America in Hollywood, Florida, on August 13 (AFP photo)
WASHINGTON — Pfizer and BioNTech said Wednesday a completed analysis of their experimental COVID-19 vaccine found it protected 95 per cent of people against the disease and announced they were applying for US emergency approval "within days".
The news from the US pharmaceutical company and its German partner brought further hope to a world upended by the coronavirus pandemic, and follows an announcement last week in which they said a preliminary analysis showed the product was 90 per cent effective.
Adding to the encouraging data was that the efficacy was found to be consistent across all age-groups — a primary concern for a disease that hits the elderly the hardest — as well as genders and ethnicities.
"The study results mark an important step in this historic eight-month journey to bring forward a vaccine capable of helping to end this devastating pandemic," said Pfizer CEO Albert Bourla.
Wednesday's news came after 170 people fell sick in an ongoing clinical trial of almost 44,000 people — 162 of whom were in a placebo group and eight of whom received the two-dose medicine.
Out of the 170 patients who became sick, 10 developed severe COVID-19 — nine in the placebo group and one in the vaccine group.
The new data showed the vaccine was generally well tolerated, with most side-effects short-lived and either mild or moderate.
About 4 per cent experienced severe fatigue and 2 per cent got severe headaches after their second dose. Older patients had fewer and milder side-effects.
The preliminary analysis announced last week had been based on data from 94 sick people, and the companies say the additional patients have now given them a more complete picture.
The ongoing late-stage trial is taking place at 150 sites in the US, Germany, Turkey, South Africa, Brazil and Argentina.
The trial will continue for another two years to find out how long immunity lasts and whether there are longer term safety issues.
If the Food and Drugs Administration issues an emergency use authorisation, the vaccine could start reaching the first Americans — frontline workers, the elderly and other high-risk people — in the first half of December.
US officials have said supply will steadily increase and it expects to have enough doses of multiple vaccines to cover the whole population by next spring or summer.
Pfizer and BioNTech said they expect to produce 50 million doses this year and 1.3 billion by the end of 2021.
Experts welcomed the news but said they awaited more detailed information, such as a breakdown of the age and underlying health of volunteers — which might explain the severe COVID-19 case in the vaccine group.
But the overall reaction was highly positive.
“How good is this?” tweeted Benhur Lee, a microbiologist at The Icahn School of Medicine at Mount Sinai, noting the efficacy compared favorably with established vaccines for Hepatitis-B and MMR (measles, mumps and rubella).
Messenger RNA
On Monday, US biotech firm Moderna said the two-dose vaccine it developed with the US National Institutes of Health (NIH) was 94.5 per cent effective, according to a preliminary analysis.
Both vaccines use mRNA (messenger ribonucleic acid) technology to deliver genetic material to the body that makes human cells create a protein from the virus.
This trains the immune system to be ready to attack if it encounters SARS-CoV-2.
“These achievements highlight the potential of mRNA as a new drug class,” BioNTech CEO Ugur Sahin said.
There are important differences between the two vaccines.
Pfizer’s needs to be stored at -70ºC while Moderna’s only needs -20ºC, more akin to a regular freezer.
This is because they have different formulations of “lipid nanoparticles” used to deliver the mRNA molecules.
The deep-freezer conditions needed for the Pfizer-BioNTech vaccine have raised logistics concerns, which the companies tried to address on Wednesday.
They said they had developed “specially designed, temperature-controlled thermal shippers utilizing dry ice to maintain temperature conditions” which can be used for up to 15 days by refilling with dry ice.
On the other hand, the Pfizer-BioNTech doses are much smaller — 30 micrograms to Moderna-NIH’s 100 micrograms — probably lowering production costs per dose.
Related Articles
WASHINGTON — US biotech firm Moderna on Monday announced its experimental vaccine against COVID-19 was almost 95 per cent effective, marking
PARIS — Russia's Sputnik V vaccine is 91.6 per cent effective against symptomatic COVID-19, according to results published in The Lancet on
WASHINGTON — The US green-lit the Pfizer-BioNTech COVID-19 vaccine late Friday, paving the way for millions of vulnerable people to receive